From drugs to medicine
Running the gauntlet: the clinical trials process
These days, cannabis is widely talked about as a medicine. It s not unusual to see newspaper articles or social media posts that promote its medical properties . But how does a drug become a medicine? And is cannabis really considered a medicine yet?
For cannabis and the chemicals extracted from it to be accepted as safe and effective medical drugs, they must first pass the same tests as any other potential medicine that might be helpful to the public. The system used to test this in Australia and around the world is the clinical trial process. Only by passing each stage of this process successfully, can a drug demonstrate it is safe and effective enough to be made available to the market and benefit the population.
This is not an easy process the average time from development of a drug to availability to patients is eight years, and on average, only 1 in 5 drugs make it through the tests successfully. For some medical conditions such as cancer, it s less than 1 in 10 drugs that make it through successfully. That means 9 out of 10 drugs that we read about in the paper as possible cures or treatments of cancer don t turn out to be safe enough or effective enough to actually be used.
So how does a drug move from an idea to a medication?
Click the arrows below to learn about each step a drug must take before it becomes a medicine.
Phase 0: Drug development / preclinical stage
The training ground
Phase 0 (also known as the preclinical stage) is like qualifications drugs have to prove their worth before they can pass on to actual human testing.
During this stage, newly developed chemicals (or recently found ones) are tested in animals or cells to find some evidence of a positive effect.
It can also be where new delivery methods are tested an old drug that was once an injection might have been turned into a pill (which patients would prefer!), but scientists need to check it still works in this new form.
In some instances, pilot studies or case studies are used these are very small trials (less than 20 people, or for case studies only one or two) used when other preclinical testing is not available.
Most of the current testing for medicinal cannabis is still in these training stages there may be some promising animal and cell data, and some interesting case studies, but usually not enough of each and that means there isn t enough evidence for it to pass to human clinical trials.
Who decides if it s ready for the next stage?
Whether a drug is ready to pass to Human Testing is decided by an ethics committee if they don t think the evidence is good enough or the risk of harm is significant, then a study can be rejected. The ethics committee will decide at each stage if a trial can continue.
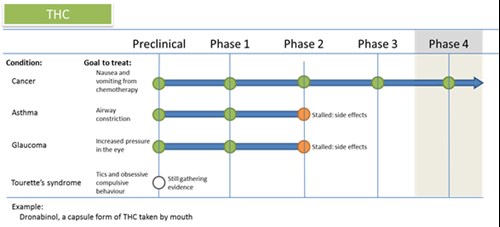
| Spotlight: THC and Tourette s syndrome Tourette s Syndrome is a disorder consisting of motor and verbal tics (sudden semi-voluntary movements or vocalisations that last a short time) which are often preceded by a sensory urge. It has been claimed cannabis can help decrease the frequency of tics. Right now, research into Tourette s Syndrome and cannabis is very limited. There have been a few pilot and case studies (which means only a few people tested) and one or two animal studies. A recent research review of the evidence has found that the results are too vague and more evidence needs to be gathered before it can move into proper human testing. So you can see, it s not easy for a drug to get past even the first stage! |
Phase 1: Early human testing
Pilot studies
The goal of phase 1 is to assess the safety of the drug in human volunteers.
We do this because drugs that work in animal models do not always have the same effects in humans a very effective drug in rats may have no effect on humans at all. Likewise, a safe dose for a mouse when made to an equivalent dose may be very dangerous for humans.
Only a small number of people are used in these studies (on average between 20 to 50 people). In most cases these are healthy humans, but in some cases a clinical patient group is used. This is most common in cancer trials where a drug may cause a healthy person to become unwell.
From this stage researchers learn: the maximum dose of a drug that can be tolerated, how long a drug takes to metabolise (break down in the body) and its basic effects (including side effects).
About two thirds of drugs pass this stage the main reason for failure is usually the drug having unexpected or serious effects in humans.
| Spotlight: Vaporisers One reason smoked cannabis has remained so popular is that breathing in a chemical is the quickest way for it to get to the bloodstream to have its effects. However, like smoking tobacco, this can have some serious side effects when plant matter is burned it creates by-products such as tar which can irritate or damage the lungs. It is unlikely, for this reason, that smoking cannabis will become the main way for patients to take medical cannabis in the future. A vaporiser is a device that heats water and cannabis and in doing this, creates a cannabis mist. Vaporisers have been suggested as an alternative method for taking cannabis as they might keep the best parts of smoking (fast effect) and limit the side effects (lung and throat irritation and/or damage). Vaporisers have been tested with small groups of humans to assess their safety. So far tests have found they do have less direct negative effect on the lungs than smoking, but more testing is needed to see if they contain other chemicals such as formaldehyde that may make it unsafe in other ways. |
Phase 2: Patient testing
 Is it better than nothing?
Is it better than nothing?
The goal of phase 2 is to make sure a drug is safe for patients but also that it actually works!
Using information from phase I, the drug is now tested in a patient population. In this stage, a larger number of people are assessed (up to several hundred) and a lot of questions have to be answered.
Patients can be quite different and sometimes many trials of the same drug have to be done in order to find the best or complete potential patient population. To be successful, the drug must produce few or tolerated side effects, and must show some benefits compared to a placebo (a treatment that should not have any effect like a pretend treatment). Study times are longer in this phase, from several months to a few years.
Only a third of drugs tested pass this 3 common reasons for failure are unwanted side effects that weren t found in phase 1 or, more commonly, being unable to prove any benefit compared to placebo.
What is a placebo?
You ll often hear the word placebo used in medical research and it s not the band your dad used to listen to in the 90s!
A placebo is a fake treatment that is given to the patient so the scientists can see if any change in a patient s condition would have happened anyway, without treatment, of if the change happened because the patient thought it would. For example, if one patient is given a tic tac (a placebo, because it has no medical use) and another is given a working drug, the patient with the placebo should get no real medical benefit. If the patient seems to report a lot of benefit it could be that simply receiving medical attention has helped, or because they know they are being treated, or they could just really want to believe that it has worked.
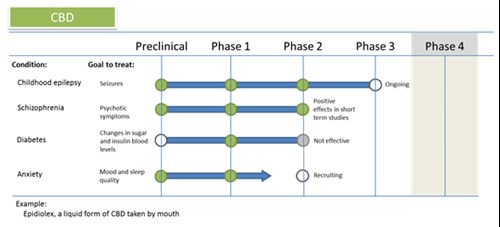
| Spotlight: CBD for Schizophrenia Schizophrenia is a mental disorder in which the sufferer can have hallucinations and confused thinking. It can also impact memory and reasoning. Current drugs to treat it can have unpleasant side effects like weight gain and trembling, and don t work for everyone. For this reason there is a lot of interest in searching for new medications. CBD, which is one chemical in cannabis, has been suggested as a possible treatment. It has passed through stage 1 testing, meaning it was safe at the doses tested. Early experiments in schizophrenic patients seem to be reducing symptoms, but there are a few problems that need to be worked out it doesn t work for all patients, and studies have so far only been short-term. |
Phase 3: Testing against other medications
Is it better than other treatments?
In this stage, the new medication has to prove it is equal to or better/cheaper than an existing treatment. Having a drug that works but only works half as well as the already available treatments is not going to do well what patient would want to use it?
You may hear the term randomised controlled trial used when talking about medical research this is the gold standard (the really good quality research) of phase 3. This uses a research design which randomly chooses which patient will get which drug, for example an old drug, the new drug being tested, or a placebo (a fake treatment that has no effect). This makes sure the scientist doesn t just choose his friends to get the new treatment, or people he knows will say good things about the drug.
A trial can be also blinded when either the doctor or the patient (or both) do not know which treatment or drug is being given to which patient. So while recording the effects, the scientist doesn t know if the patient in front of him took a real drug, a pretend one, an old one or the drug being tested they just see what is happening to the patient.
In this stage, the number of tested patients gets larger; thousands of people can be involved in a phase 3 trial. The trial times are also much longer to test for long-term effects. For this reason it can take several years before a result is known.
Drugs are tested against placebo as well as against an already known treatment. In this way researchers can see whether the new drug works more like the placebo (which should have little effect), like the existing/old drug, or better than both.
In both phase 2 and phase 3 the reasons for failure are similar thanks to testing in phase 1 and 2, drugs at this stage are should be fairly safe, so drugs usually fail because they aren t effective.
| Spotlight: CBD and childhood Epilepsy In recent years there have been a lot of media attention about the theory that CBD (a chemical in cannabis) can reduce seizures in children with epilepsy. There is a lot more attention on epilepsy now and research has sped up to the stage where drug trials are now in phase 3 trials. While Phase 2 results were promising, a small percentage of patients who took CBD found their seizures got worse, and for some there was no change at all. The goal now is to find out why patients respond differently. The more that is known about how the drug works and why, the better people will be treated in the future. |
What happens after Phase 3?
If positive results are found in Phase 3, there may be enough evidence for the drug to be available to the public. But there is one final hurdle approval from a government regulatory body.
In Australia, the organisation that approves drugs for public use is called the Therapeutic Goods Administration (TGA). The TGA s job is to make sure the benefits of a new drug outweigh the risks.
Drugs are often products that are made by big companies and they can cost people a lot of money to buy. That means they can also make a lot of money for the big companies that own them. For this reason, we can t just let those big companies test the drugs themselves and then make them available they could just say they work, even if they don t, because they want to make money.
The TGA assesses the results from Phase 1, 2 and 3 clinical trials to make sure the drug meets the standard set by the Australian Department of Health. They are also in charge of how the public will have access to the new medication whether it can be in the supermarket, in a pharmacy or only by special request. If there is enough evidence it is effective and safe, they will make it available to the public.
Phase 4: Marketed and further research
Acceptance and beyond
Success! The drug has made it through Phase 1, 2 and 3 and is available to the public. But there is no rest yet there is more research to be done!
This research is done by a range of different international research groups in clinical settings, rather than within specialist research facilities, to ensure the findings are very robust and reproducible. This may be done alongside this next phase.
This stage is known as Phase 4, or the post-marketing phase. The new drug will now reach thousands or potentially millions of people, and with this amount of patients, it is now possible to search for very rare side effects.
This is also the time to look for unexpected drug interactions (negative side effects caused by the drug mixing with other things, like other drugs or foods) there are thousands of medications taken daily, and it would be impossible to test them all in the clinical trial phases.
By gathering information in this stage, the drug can then be made safer by identifying which patients have the best response to the drug and which patients should avoid it. It can also reveal other therapeutic effects not related to the drug s original design if this is the case, the drug s use can be expanded (provided it passes phase II and III again for this new condition, of course).
| Spotlight: Rimonabant Medications can be withdrawn from public sale if side effects are found that weren t detected in the early stages of testing. This has happened to Rimonabant, a cannabinoid drug that was designed to treat obesity and passed through the clinical trial process in Europe. When it was made available to the public it was found to cause seizures and psychiatric events in some patients these were serious enough that it was withdrawn from the market after two years. |
What happens if the drug fails to run the gauntlet?

| Knocked out: THC for glaucoma THC has been known to treat glaucoma, an eye condition which can cause eventual blindness, since the 1980s. However, it has not yet been able to overcome some serious downsides to make it a good medication: 1) It doesn t last long enough, meaning patients would need too many doses to be manageable (they d need to use it around 8 times each day!). 2) It also causes unwanted side effects that patients don t like, including psychoactive and cardiovascular effects. Researchers are currently trying to find a way to make it last longer and have its effects only in the eye eyedrops have been tested but aren t yet perfected. There are also much more effective drugs available. Back to phase 1! |
What happens when you don t use the clinical trial system?
The clinical trial process has the downsides of being slow and expensive. You might wonder what s the point, especially when people have been using cannabis for years?
The reason the clinical trial process is so important is it helps us better understand a drug how safe it is, how we should use it, what side effects we can expect, just to name a few. It also helps stop big companies from just releasing dangerous drugs to make quick profits.
It is generally a bad idea to blindly trust the health claims of someone who has something to gain from you buying their product this is true of pharmaceutical companies, but it is also true of food producers, health care product makers, celebrities and cannabis sellers. It would be very tempting for a seller to oversell the good of their product and hide/ignore the bad. The law of selling used to be Caveat Emptor: in English this is let the buyer beware or in other words, buy at your own risk!
The only way to make sure people or businesses selling a product are being held accountable is regulation – the clinical trial procedure is a way to keep up the standard of pharmaceuticals, but also provides monitoring specifically to make sure it is both safe and effective.
The standard for therapeutic goods that aren t counted as pharmaceuticals, things like herbal remedies you buy at the supermarket, is much less difficult to pass than the phases you ve just learned about. Businesses selling these types of goods are only required to prove that what they are selling is safe it doesn t necessarily have to be effective. So you might be paying for nothing!
These products often have lots of ingredients, making it very difficult to know what might be beneficial (or if the ingredients cancel each other out). Long-term monitoring of use and drug interaction studies are also at a much lower standard it is only when bad effects are reported in the public enough that a product can be found unsafe. This may be too late for those who have taken something hoping to improve their health. And this is only if you are taking an Australian drug if you are buying something online, it might not be regulated at all!
In the United States it looks like some cannabis products are not reaching the standard required for pharmaceuticals a study looking at the accuracy of labelling for edible cannabis products in California found that less than 20% of them actually had the amount of THC they claimed to contain. As a buyer, it becomes difficult to have faith in a product if you can t trust what is written on the label.
Where are we up to with cannabis?
There s still a lot of research to be done for cannabis as a medicine. Unfortunately a lot of newspapers and online media forget this, and publish headlines like cannabis cures cancer before the drug has even passed phase 1. Most of the time these stories will be based on reports from preclinical studies, which is great but there is still a lot more work to do as you now know! By going through this process we can know that when cannabis does reach the public it will be as safe and effective as we can make it.
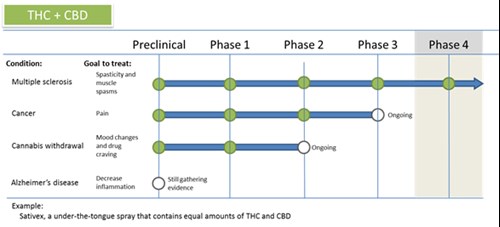
It is also important to be aware that scientists are often testing different parts of cannabis and different combinations of parts of cannabis. Cannabis is made up of more than 100 compounds called cannabinoids (for example, CBD and THC), and it seems many of these have different effects. For example, a cannabinoid called THC can make you feel high, but one called CBD probably won t. While CBD alone may be great to treat a condition, THC might make the condition worse so scientists will often pull out the CBD for use and get rid of the THC (or vice versa). This also means that just smoking a joint likely won t have the medical affects you want, and could make an illness or symptom worse.
Really reading the research, not the newspaper story is also important right now when you hear cannabinoids are used to treat cancer, they are used to treat symptoms associated with cancer (pain and nausea) rather than the cause (tumours). This might change in the future, but it always pays to get the details.



 Is it better than nothing?
Is it better than nothing?